2009
Propelling the Understanding of Adaptive Immunity

Dr. Harlan Robins, professor at the Fred Hutchinson Cancer Research Institute, and colleagues develop the first immunosequencing technology to profile the T-cell immune repertoire at scale and with precision, which had never before been possible.
2010
Unveiling the Brilliance of the Adaptive Immune System

Adaptive launches its first research product, immunoSEQ. Scientists worldwide now have this sophisticated tool to profile and monitor the adaptive immune response, to propel their research in many diseases, and to understand the immune response to therapies.
“We have delivered the Hubble Telescope as compared to a pair of binoculars to look at the adaptive immune system.”
Chad Robins, Chief Executive Officer, Co-founder, Chairman of the Board
2012
A New HQ in One of the World’s Biggest Tech Hubs

Adaptive sets up shop in South Lake Union, in the heart of Seattle.
2014
Adaptive Reaches 50 Publications

Adaptive’s technology propels research forward in oncology, hematology, infectious disease, transplantation, and autoimmunity, enabling a paradigm shift in understanding human immunology.
2015
A Giant Step Forward in Developing Clinical Diagnostics and Therapeutics
Adaptive develops new technology to map T cells to the antigens they target, providing the capability to see what diseases the immune system responds to and setting the stage for novel diagnostic tests. New technology to pair the chains of T-cell receptors (TCRs) helps identify which ones have the greatest therapeutic potential.
Honored Among Goldman Sachs’ 100 Most Intriguing Entrepreneurs

Chad and Harlan are honored by Goldman Sachs for their groundbreaking technology and the promise Adaptive can bring to the future of medicine.
100 Publications

Scientists across disease areas continue to propel their research, elucidating the immune response in diseases as varied as multiple sclerosis, metastatic melanoma, and peanut allergy.
2016
200 Publications and Counting

Adaptive reaches 200 publications based on our technology.
2017
Adaptive Partners with Microsoft to Map the Human Immune System

Adaptive partners with Microsoft, combining their AI and machine-learning capabilities with our platform to map TCRs to the diseases they detect at scale. The goal is to develop blood tests for early and accurate detection of many diseases.
2018
First Immunosequencing–Based Diagnostic Cleared by FDA
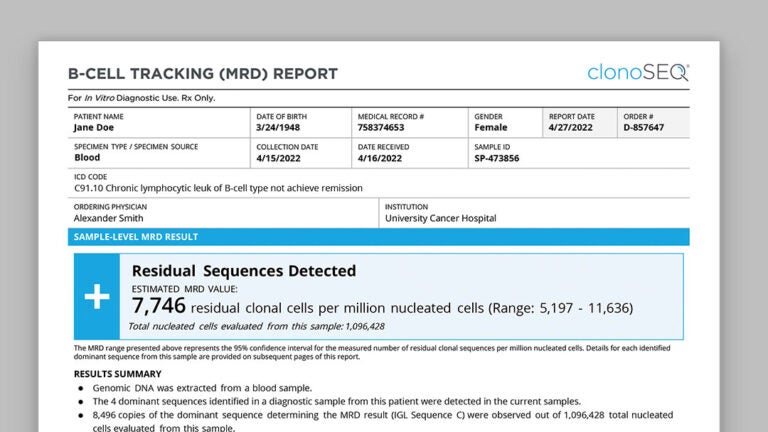
clonoSEQ receives FDA clearance for the detection and monitoring of measurable residual disease (MRD) in patients with multiple myeloma and acute lymphoblastic leukemia.
2019
Adaptive and Genentech Partner to Develop Personalized Cellular Therapies
Adaptive and Genentech join forces to develop shared and personalized T cell–based therapies for cancer patients, validating the therapeutic potential of Adaptive’s platform.
clonoSEQ Covered by Medicare

Patients with certain blood cancers are ensured access by Medicare for MRD assessment to support more personalized treatment decisions. This paves the way for further coverage by private payers.
Adaptive Goes Public

In one of the biggest biotech IPOs of the year, Harlan and Chad Robins ring the Nasdaq opening bell as Adaptive is listed under the ticker symbol ADPT.
2020
500 Publications and Building

From narcolepsy to ovarian cancer. From HIV to SARS-CoV-2. From the healthy baseline of the immune repertoire to kidney transplantation. Adaptive continues to propel the understanding of the smallest details and the biggest insights of immune response.
Decoding the Immune Response to COVID-19
Adaptive, in partnership with Microsoft, points its platform against COVID-19. They share the data freely with researchers around the world to inform novel diagnostics, therapeutics, and vaccines via ImmuneCODE, the largest database ever developed of T-cell immune responses to this virus.
Enabling a New Way to Measure Immunity

Adaptive launches immunoSEQ T-MAP COVID, a new tool for researchers that measures the T-cell response to vaccines in development and tracks the persistence of the responses over time.
clonoSEQ Expands to Third Indication for Chronic Lymphocytic Leukemia

For the first time, the FDA clears clonoSEQ for use as a blood-based test. Patients living with the most common type of leukemia have a new and convenient way to get answers about their disease and remission status.
The First-Ever T Cell–Based Diagnostic Test

Adaptive launches T-Detect COVID, a novel diagnostic test for patients that uses the T cell as a marker to indicate recent or past infection. This is a small step for Adaptive and a large step towards Adaptive’s and Microsoft’s ultimate vision to develop T cell–based tests for early and accurate detection of many diseases from a single blood sample.
What's Next
The Future of Immune Medicine

Adaptive is powering the age of immune medicine and propelling a paradigm shift in healthcare. The combination of our unique approach and the near-infinite applications of immune medicine provide opportunities to make a difference in the lives of people living with many different diseases, both now and in the future.
This is just the beginning of the story.

