MRD Biopharma Services
The power of MRD testing in clinical trials
clonoSEQ MRD testing: Enhancing insights in clinical trials data
Due to transformative therapeutic advancements, patients with lymphoid malignancies are now achieving deeper responses and significantly better outcomes. As a result, clinical trials for new therapies using traditional response measures can take 10-15 years to complete, thereby delaying access to innovative and more effective treatments.
Using clonoSEQ to measure and monitor minimal residual disease (MRD) enables the detection of small yet significant levels of disease. The assay can be a crucial tool to inform drug performance, provide an earlier read on efficacy, and offer a clearer understanding of the depth and duration of response to novel therapies.
clonoSEQ MRD testing in lymphoid cancers
Early and precise read on efficacy
Highly sensitive and specific
Quantitative results
ID disease missed by other measures of response
Used by world’s leading drug developers

Early and precise read on efficacy

Highly sensitive and specific

Quantitative results

ID disease missed by other measures of response

Used by world’s leading drug developers
Unmatched MRD expertise and proven utility
Valuable data and insights throughout the drug development process
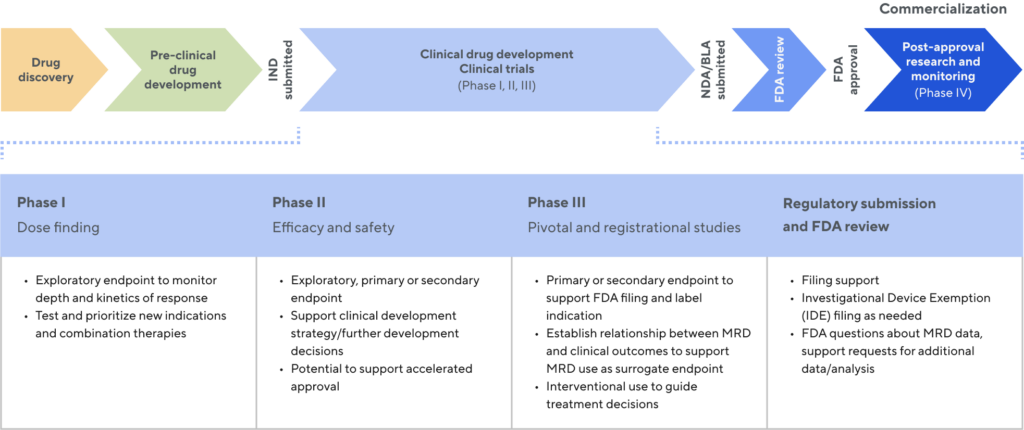
Experts in MRD-informed clinical trials
Clinical trial design
Post-hoc analysis

Global regulatory strategy
Supported by more than 150 publications

Clinical trial design

Post-hoc analysis

Global regulatory strategy

Supported by more than 150 publications
Deep sensitivity and validated performance
Detects one in one million cancer cells

Standardized MRD results across all global sites

CE-marked under the European Union’s In Vitro Diagnostic Medical Device Regulation
U.S. regulatory approval
| Disease state | FDA-cleared | CLIA-validated and CLEP approved |
|---|---|---|
| Acute lymphoblastic leukemia (ALL) | ||
| Chronic lymphocytic leukemia (CLL) | ||
| Diffuse large B-cell lymphoma (DLBCL) | ||
| Follicular lymphoma (FL) | ||
| Mantle cell lymphoma (MCL) | ||
| Multiple myeloma | ||
| Other non-Hodgkin’s lymphomas |

Flexible sample types and timepoints*
- Fresh or frozen specimens
- Cellular and circulating tumor DNA (ctDNA)
- Bone marrow, blood, plasma, and tissue
Global clinical trials and regulatory experience
- More than 40 pharma partners and 160+ active industry-sponsored clinical trials
- Secondary endpoint in more than 70 active studies, primary endpoint in 10+ active studies
- Data generated using clonoSEQ has supported regulatory approvals for various therapeutic modalities, including CAR T, bispecific T-cell engagers, monoclonal antibodies, and BCL2 inhibitors
- Successfully supported Biopharma partners in more than ten Human Genetics Resources Administration of China (HGRAC) applications


Widely adopted in-clinic
- In NCCN guidelines and used in clinical practice by all 33 NCCN cancer centers
- Ordered by 4,100 providers for more than 60,000 patients
- Broad U.S. payer coverage: over 300 million covered lives (MM and ALL); nearly 200 million covered lives (CLL); over 65 million covered lives (DLBCL and MCL)
How it works
clonoSEQ is a tumor-informed assay

Step 1: ID test
- Conducted once using a high tumor burden sample
- Identifies trackable immune receptor DNA sequences associated with a lymphoid malignancy

Step 2: MRD tests
- Conducted at any timepoint
- Detects and quantifies the sequence(s) identified in step 1 during or after treatment
- Even at very low levels, immune receptor DNA sequences associated with a lymphoid malignancy can be detected
Select Publications
clonoSEQ assay technology has helped Biopharma partners prioritize and advance therapeutic assets for lymphoid malignancies for more than a decade and has been included in more than 150 publications.
CLL
DLBCL
Current clients, contact the Alliance Management team at alliancemanagers@adaptivebiotech.com.
* For accepted sample types for each disease state, see the Specimen Guidelines for GCLP Batch Workflow Analysis.
clonoSEQ® is available as an FDA-cleared in vitro diagnostic (IVD) test service provided by Adaptive Biotechnologies to detect measurable residual disease (MRD) in bone marrow from patients with multiple myeloma or B-cell acute lymphoblastic leukemia (B-ALL) and blood or bone marrow from patients with chronic lymphocytic leukemia (CLL). clonoSEQ is also available for use in other lymphoid cancers and specimen types as a CLIA-validated laboratory developed test (LDT). To review the FDA-cleared uses of clonoSEQ, visit clonoSEQ.com/technical-summary.