In light of the COVID-19 pandemic, understanding the role of the immune system has become critical in deciphering how this highly contagious virus affects people across the globe of all ages, ethnic backgrounds, and social backgrounds, with widely varying responses. This has unequivocally brought the role of the immune system to the forefront of research and the public discussion, making Adaptive’s immune medicine platform more relevant than ever. We are seeing that with this virus, as with many other pathogens, the T cell provides a key link to understanding exposure and immunity when it comes to COVID-19. Now we have the ability to use T cells to detect SARS-CoV-2 and leverage this capability to measure and monitor a more comprehensive immune response to vaccines in development.
Critical role of the T cell in the immune response to SARS-CoV-2
We and others are learning a great deal about the critical role T cells play in the immune response to SARS-CoV-2. Specifically, exposure to the novel coronavirus can induce virus-specific T-cell responses without inducing antibodies and can be detected up to 100 days after symptom onset, the maximum time period currently available to assess response,1 as shown in this diagram below.
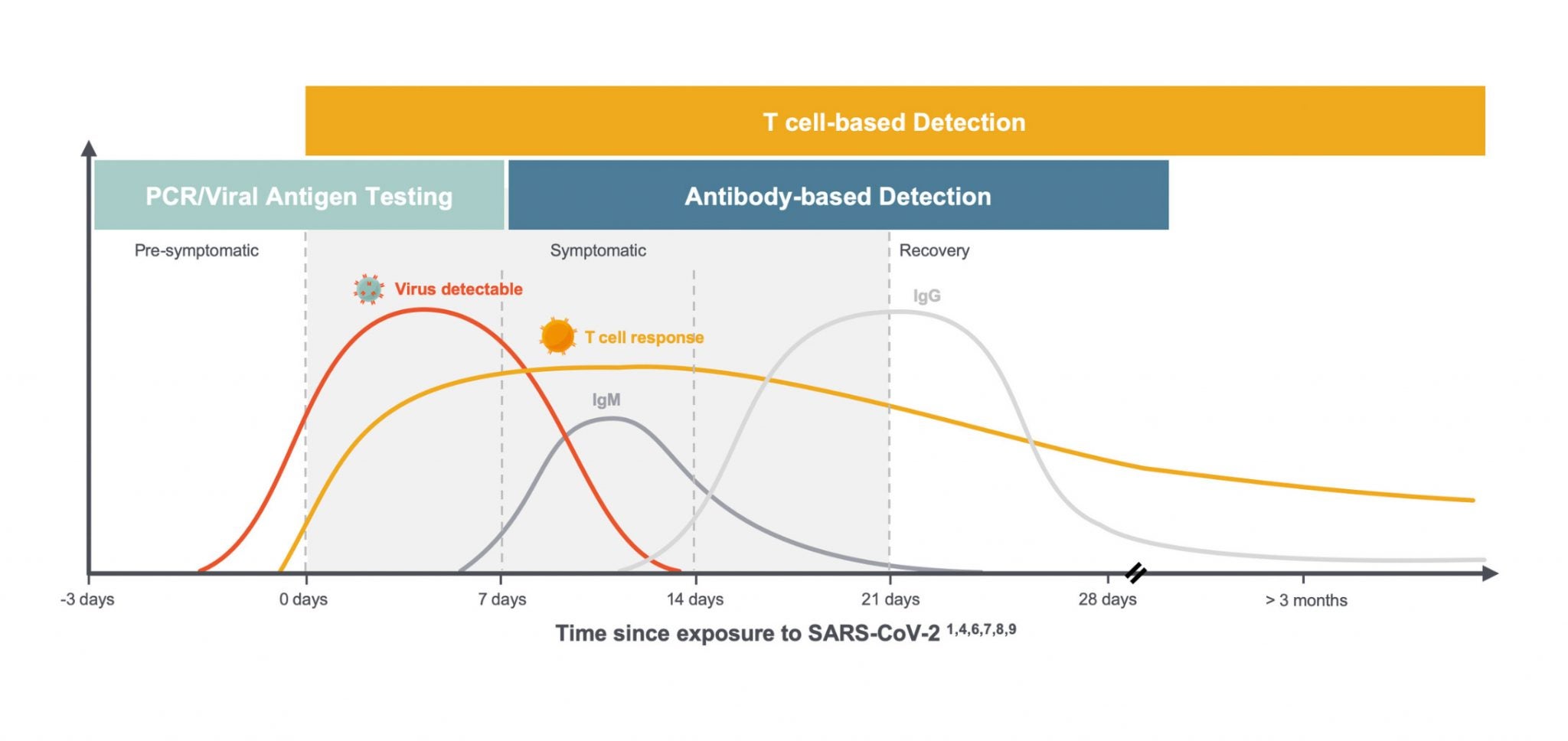
Much of what we are learning about T cells is consistent with what we see in the immune response to other viruses, but the combination of global scientific focus and the number of people infected with the virus in such a short period of time has led to an understanding of the variability of individual responses on a greater scale. In the setting of COVID-19, we are seeing:
- T-cell responses arise earlier than antibodies and last through clearance into convalescence. 2
- T cells play a critical role in supporting the development of antibodies by B cells and can serve as the first signs of an immune response to SARS-CoV-2 infection. 3
- The majority of COVID-19 patients generate a T-cell response comprised of both CD8+ T cells, or “killer” T cells which destroy virus-infected cells, and CD4+ “helper” T cells, which help other immune cells, including B cells which produce antibodies. 5
- CD8+ & CD4+ T cells were observed in convalescent patients with mild and severe COVID-19 disease. 6
Solving a big data problem to study the T cell – the critical missing link in immunity
What makes the task seem daunting is that T cells have been historically notoriously hard to study. Our bodies have hundreds of millions of different T cells in order to be prepared to respond to millions of different types of threats. Most researchers have continued to rely on techniques to either measure the immune response solely based on antibody levels, or sometimes measuring T cells using techniques that are expensive, bespoke and low throughput.
At Adaptive, we set out to solve this problem several years ago. Our platform enables us to identify the specific T cells that map to any disease quantitatively at unprecedented scale, speed and precision. Soon after the pandemic hit, our team at Adaptive Biotechnologies, along with our partners at Microsoft, worked quickly to map the T-cell response to SARS-CoV-2 across the population to make these data freely available via ImmuneCODE, which we believe to be the largest database for the T-cell immune response to this virus.
To date, we have analyzed over 1,400 de-identified patient samples. From these samples, we have identified over 135,000 T-cell receptors (TCRs) that map not only to the spike protein but to 10 other specific parts of the virus which we have shown to be most likely to induce an immune response. It is our hope that these data will help researchers around the world in their quest for solutions to this pandemic.
In addition to making these data publicly available, we have developed two applications: a T-cell based diagnostic, and a T-cell monitoring tool for vaccine developers.
Benefits of using T cells to detect SARS-CoV-2
Using a set of identified “shared” TCRs that are seen across multiple infected individuals, we identified a T-cell signature that can be used for diagnostic purposes. Data from Adaptive Biotechnologies and from others to date show that the properties of T cells, outlined above, could make a test that detects virus-specific T cells a more effective way to assess SARS-CoV-2 exposure before antibodies arise and after they wane.
Comparing T-cell signature with antibody serology
To demonstrate this, we recently conducted a head-to-head study in a real-world setting comparing our test under development with two leading serology tests in 100 patients, ranging from active infection through convalescence. The tests were set at 99.8% specificity to minimize false positives. In this study, 94% of patients were detected as positive by Adaptive’s T-cell test under development vs. 90% (IgG and IgM) and 87% (IgG only) for the serology tests, respectively.1
What we are learning about immune response to this virus from our own studies and other research highlight three reasons that may explain the higher sensitivity of a T-cell test:
- Patients could be tested after the T-cell response begins but prior to seroconversion. Two of the participants who originally enrolled in our ImmuneRACE study as “exposed” subjects became acutely infected prior to sample collection tested positive for T cells but not for antibodies.
- Some patients may effectively fight the virus with T cells alone and never seroconvert. One T-cell positive patient in our 100 sample cohort was asymptomatic with two positive PCR tests, but tested negative by both antibody tests. Other researchers have also reported infections that lead to T-cell response but no detectable antibody response.4
- Antibodies wane over time. In our data, we have seen persistence of T cells for most patients as far out as we have measured (~100 days). Other studies have demonstrated that, in contrast, antibodies seem to wane over time although we have not yet observed this in our own data. Literature shows in another coronavirus infection, SARS-CoV-1, that virus specific T cells have been routinely detected in studies in the years following the initial SARS outbreak, including at least a decade after initial infection, while antibodies waned quickly. 10,11
These results, together with a growing body of evidence indicating T cells can be present in the absence of antibodies, suggest the potential utility of Adaptive’s T-cell based approach to detect immune response to SARS-CoV-2 earlier, and in less severe cases, than tests that detect antibody response.
Regulatory submission
Detection of recent or past infection is our first application, and we are currently working with the FDA to generate and package the data required to submit for Emergency Use Authorization (EUA). We anticipate that the data we continue to generate will expand the clinical applications for our T-cell based testing approach to potentially include assessing pre-existing immunity based on cross-reactive T cells, post infection immunity, and immunity from a vaccine, which may need to be monitored for possible boosters over time.
The need to study T cells when measuring and monitoring immune response to vaccines
As we learn more about the role T cells play in immunity, vaccine developers are recognizing the need to measure the T-cell response in addition to the antibody response to their vaccines. The same qualities that make a T-cell test ideal for diagnostic purposes also underscore its utility for measuring immune response to vaccines. We recently launched immunoSEQ® T-MAP™ COVID as a tool to offer vaccine developers a way to integrate our map of SARS-CoV-2-specific T cells into their vaccine trials.
This is the first molecular T-cell monitoring tool for SARS-CoV-2 that accurately, quantitatively, and reproducibly measures the T-cell immune response to vaccines and tracks the persistence of that response over time. We are providing data that map those TCRs to SARS-CoV-2 antigens, a capability that may significantly improve the ability to measure the immune response to vaccines in development. Importantly, we can do this from a simple blood sample that does not require any special storage or handling.
Speed and samples are needed to learn more
Globally, we are all moving quickly to learn more about the virus and find solutions that will help us turn the corner in this fight. We believe that T cells are key to understanding exposure and immunity – one of the many important pieces of this very big puzzle. Our focus remains clear. We will continue to map T cells at scale, track their immune response to vaccines in development, and track their persistence over time, working together in this collective cause to help end this pandemic. If you are interested in contributing to this important effort: Institutions or collaborators interested in contributing blood samples can direct inquiries to COVID19ImmuneResponse@adaptivebiotech.com, and our ImmuneRACE study is still recruiting – visit www.ImmuneRACE.com for more information.
- Snyder, et al MedRxiv preprint, 2020
- Funk, et al. Frontiers in Pharmacology, 2020
- Sekine, et al. BioRxiv preprint, 2020
- Gallais, et al. MedRxiv preprint, 2020
- Grifoni, et al. Cell, 2020
- Peng, et al. BioRxiv preprint, 2020
- Paolo, et al. Pediatric Allergy & Immunology, 2020
- Subbarao, et al. Immunity, 2020
- Channappanavar, et al. Immunol Res, 2014
- Tang et al, J Immunol, 2011
- Ng et al, Vaccine, 2016
